The TIM-3 Alzheimer treatment embodies a groundbreaking approach that harnesses the immune system to combat Alzheimer’s disease, particularly in its late-onset form, which constitutes the majority of cases. Researchers have recently highlighted the pivotal role of TIM-3, an immune checkpoint molecule, in regulating the activity of brain immune cells known as microglia. By silencing TIM-3’s inhibitory effects, scientists were able to enhance the ability of microglia to clear harmful amyloid plaques from the brain, thereby improving cognitive function in animal models. This innovative strategy, initially developed for cancer therapy, opens up new possibilities for treating Alzheimer’s, a condition that has long resisted effective solutions. As research progresses, the potential for TIM-3 therapy to reshape the landscape of Alzheimer’s treatment appears increasingly promising.
Exploring innovative therapies for Alzheimer’s disease, the TIM-3 treatment represents an advanced strategy that aims to alleviate symptoms by targeting the immune system. In this context, the focus is on the TIM-3 molecule, which acts as a molecular checkpoint, governing the microglial response in the brain. By inhibiting TIM-3 activity, researchers can boost the microglial clearance of beta-amyloid plaques, thereby restoring cognitive abilities in affected individuals. This concept draws parallels to existing cancer treatment methodologies, where immune modulation has yielded promising results. As scientists delve deeper into this relationship between immune functioning and neurodegeneration, the prospects for effective Alzheimer therapies continue to expand.
Understanding Alzheimer’s Disease and Its Challenges
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that primarily affects the elderly population, accounting for approximately 90-95% of all cases. Characterized by memory loss, cognitive decline, and behavioral changes, AD poses immense challenges not just for those diagnosed, but also for caregivers and healthcare systems. Current treatments offer only symptomatic relief, highlighting the urgent need for innovative therapeutic approaches that address the underlying mechanisms of the disease.
Recent research has revealed significant biological factors contributing to Alzheimer’s, particularly the role of amyloid plaques and tau tangles in the brain. These abnormal protein accumulations disrupt neuronal function and communication, leading to the characteristic symptoms of memory impairment and confusion. Immunotherapy targeting these plaques is garnering attention, as understanding the immune response to such accumulations may unlock new treatment modalities.
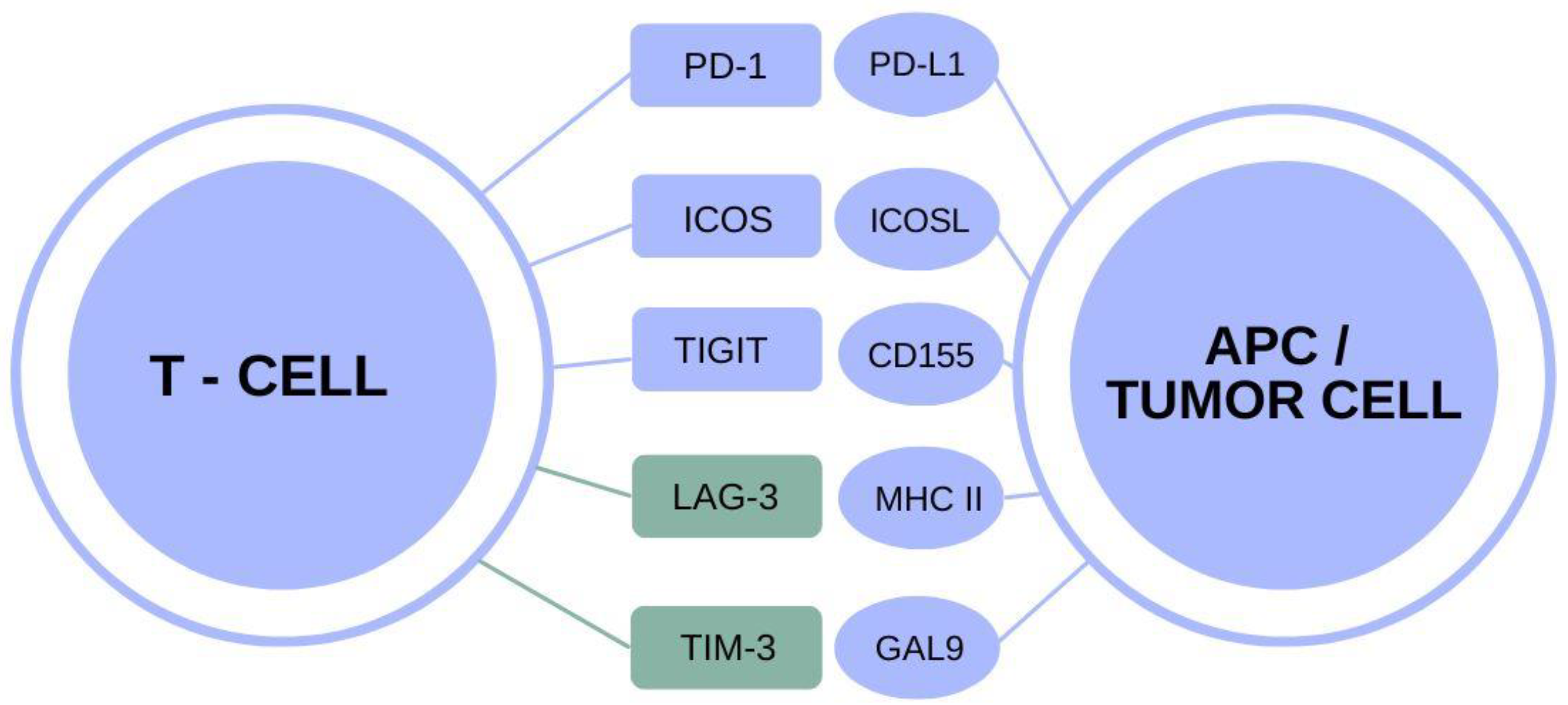
The Role of TIM-3 in Alzheimer’s Disease Treatment
TIM-3, or T-cell immunoglobulin domain and mucin domain-3, has emerged as a pivotal checkpoint molecule in the immune system’s regulation, particularly concerning Alzheimer’s disease. Research indicates that elevated expression of TIM-3 on microglia, the brain’s resident immune cells, prevents the clearance of amyloid-beta plaques. This inhibition may contribute to the advancement of cognitive decline in patients with Alzheimer’s, underscoring the need to target TIM-3 for potential therapeutic intervention.
Preliminary studies demonstrate that deleting the TIM-3 gene in mouse models enhances microglial activity, allowing for better clearance of plaques and improved cognitive function. These findings suggest that TIM-3 could be a strategic target for Alzheimer’s treatment, with therapies designed to inhibit its function potentially reversing memory impairment and enhancing overall cognitive health in affected individuals.
Microglia: Guardians of Brain Health
Microglia play an essential role in maintaining brain health by clearing cellular debris and modulating inflammation. During the early stages of Alzheimer’s disease, microglia are activated to respond to amyloid plaque accumulation. However, with the expression of TIM-3 increasing, these immune cells can become dysfunctional, failing to eliminate harmful plaques that contribute to neurodegeneration.
As microglia cease their protective functions, the brain becomes increasingly susceptible to damage. By promoting the activation of microglial cells, therapies targeting TIM-3 may restore their ability to remove plaques, thereby addressing one of the key pathological hallmarks of Alzheimer’s. The balance between activation and inhibition in microglia is crucial for cognitive maintenance, and TIM-3 modulation could tip this balance back toward health.
Checkpoint Molecules: Bridging Cancer Therapy and Alzheimer’s Treatment
The concept of checkpoint molecules, such as TIM-3, gaining traction in cancer therapy is now being explored within the context of Alzheimer’s disease. In oncology, checkpoint inhibitors are used to release immune cells from self-imposed paralysis to enable the body’s natural defenses to target and eradicate tumors. A similar strategy could be applied to Alzheimer’s, where altering TIM-3’s influence on microglial cells might restore their ability to clear amyloid plaques effectively.
Repurposing cancer therapies, specifically those targeting TIM-3, holds promise for innovation in Alzheimer’s treatment. Evidence suggests that existing anti-TIM-3 antibodies may be adapted for use against the cognitive dysfunction caused by Alzheimer’s, representing a potential leap forward in therapeutic strategies that leverage the body’s immune response.
Future Directions: Transforming Alzheimer’s Disease Drug Development
The future of Alzheimer’s disease drug development may significantly hinge on adopting strategies from oncology, particularly involving TIM-3. With the exploration of therapies aimed at blocking TIM-3’s inhibitory effects, researchers are not only looking at modifying immune response but also improving cognitive function and memory retrieval in patients.
As scientists continue to investigate TIM-3’s role and its link to genetic risk factors in Alzheimer’s, prospective clinical trials are poised to take shape. Testing anti-TIM-3 antibodies in humanized mouse models could pave the way for personalized treatments, potentially leading to breakthroughs in how Alzheimer’s is managed and treated.
The Significance of Genetic Research in Alzheimer’s Disease
Understanding genetic variations that predispose individuals to Alzheimer’s disease sheds light on the complex mechanisms underlying this debilitating condition. Among these variations is the polymorphism in the TIM-3 gene, which has been identified as a significant risk factor for developing late-onset Alzheimer’s. Investigating how such genetic factors influence immune responses opens avenues for targeted therapies that harness the body’s natural defenses.
Genetic research linking TIM-3 and late-onset Alzheimer’s provides a foundational understanding of not only the disease’s etiology but also potential biomarkers for diagnosis and therapeutic targets. By comprehensively mapping the genetic landscape, future therapeutic interventions can be tailored more effectively to target specific pathways altered in individuals with Alzheimer’s.
Innovative Therapeutic Approaches to Alzheimer’s Disease
Innovative approaches to treating Alzheimer’s disease are critical as traditional therapies have largely fallen short. The recognition of TIM-3’s role as an immune checkpoint in Alzheimer’s highlights the need for therapies that can effectively manipulate immune responses to target amyloid plaques. Researchers are actively exploring the potential of small molecules and antibodies that inhibit TIM-3 to unlock a new frontier in Alzheimer’s therapy.
Developing innovative treatments requires interdisciplinary collaboration, merging insights from neurology, immunology, and pharmacology. By identifying and synthesizing potential TIM-3 inhibitors, researchers aim to create targeted therapies that not only improve cognitive function but also address the inflammatory and pathological processes central to Alzheimer’s disease progression.
Challenges in Translating Research into Clinical Practice
While research into TIM-3 and its implications for Alzheimer’s treatment is progressing, translating these findings into clinical practice poses significant challenges. One primary concern is ensuring the efficacy and safety of TIM-3 inhibitors in human patients, as the immune system’s delicate balance needs to be meticulously managed to avoid unintended consequences.
Moreover, the path from bench to bedside is often fraught with obstacles, including the lengthy process of clinical trials, regulatory considerations, and the need for comprehensive understanding of dosing and administration protocols. However, the potential benefits are substantial, and the urgency to find effective Alzheimer’s treatments will continue to drive research efforts.
Implications of Immunotherapy for Neurodegenerative Disorders
The exploration of immune system modulation in treating Alzheimer’s disease signifies a broader paradigm shift towards immunotherapy in neurology. Recognizing that neurodegenerative disorders might share common immunological mechanisms illuminates the potential for developing crossover treatments for diseases like Alzheimer’s and other forms of dementia.
As research on TIM-3 expands, the implications of these findings could extend beyond Alzheimer’s, affecting how we understand and treat a spectrum of neurodegenerative conditions. This innovative approach could herald a new era in treating diseases that have plagued medical science for decades.
The Future of Alzheimer’s Research: A Paradigm Shift
As we continue to deepen our understanding of Alzheimer’s disease through studies on molecules like TIM-3, the research landscape is witnessing a paradigm shift. Traditional views of the immune system’s role in neurodegeneration are evolving into a more nuanced perspective, emphasizing the interplay between immune checkpoints and neuroinflammation.
This shift offers hope that forthcoming therapies could significantly enhance cognitive function and quality of life for Alzheimer’s patients. By embracing innovative approaches that leverage the immune system’s capabilities, researchers are paving the way for a future where Alzheimer’s may be managed more effectively, resulting in better health outcomes.
Frequently Asked Questions
What is TIM-3 and how does it relate to Alzheimer’s disease?
TIM-3, or T-cell Immunoglobulin and Mucin domain-containing protein 3, is an immune checkpoint molecule that regulates the immune response. In the context of Alzheimer’s disease, TIM-3 is linked to late-onset Alzheimer’s because its increased expression on microglia inhibits their ability to clear amyloid plaques, which are characteristic of this neurodegenerative disease.
How does TIM-3 affect the immune system’s response in Alzheimer’s patients?
In Alzheimer’s patients, TIM-3 inhibits the microglia, the brain’s immune cells, from attacking and clearing harmful amyloid plaques. This inhibition leads to plaque accumulation, which negatively impacts cognitive function and contributes to the progression of Alzheimer’s disease.
Can TIM-3 be targeted for new Alzheimer’s treatments?
Yes, targeting TIM-3 with therapies, such as anti-TIM-3 antibodies, shows promise for treating Alzheimer’s disease. By blocking the inhibitory effects of TIM-3, therapy may restore microglial function and enhance the clearance of amyloid plaques, potentially improving cognitive function and slowing disease progression.
What were the findings of the recent study on TIM-3 and Alzheimer’s treatment?
The recent study demonstrated that deleting TIM-3 from microglia in mouse models improved memory and cognition by allowing these immune cells to more effectively clear amyloid plaques from the brain. This suggests that TIM-3 represents a viable target for new therapeutic strategies in Alzheimer’s treatment.
How prevalent is TIM-3 as a genetic risk factor in Alzheimer’s disease?
TIM-3 has been identified as a genetic risk factor for late-onset Alzheimer’s disease, with studies showing a significant association between a polymorphism in the TIM-3 gene and the likelihood of developing the disease. Approximately 90-95% of Alzheimer’s cases are late-onset, making this research particularly relevant.
What role do microglia play in Alzheimer’s disease related to TIM-3?
Microglia are essential for maintaining brain health, including clearing away amyloid plaques. In Alzheimer’s disease, TIM-3 expression on microglia inhibits their function, preventing them from clearing these plaques, which worsens cognitive decline. The study’s findings highlight the potential to restore microglial function through TIM-3 modulation.
What might a TIM-3 therapy for Alzheimer’s disease look like?
A potential TIM-3 therapy for Alzheimer’s disease could involve the administration of anti-TIM-3 antibodies or small molecules that inhibit the effects of TIM-3. This would enable microglia to effectively engage and clear amyloid plaques, potentially reversing some cognitive deficits associated with Alzheimer’s.
What are the implications of TIM-3 research for future Alzheimer’s treatments?
The research into TIM-3 and its role in Alzheimer’s disease has significant implications for future treatments. By utilizing existing anti-TIM-3 antibodies, researchers may develop new therapies that can enhance plaque clearance and improve cognitive function in Alzheimer’s patients, offering hope where previous treatments have failed.
How long has the TIM-3 Alzheimer’s research been ongoing?
The research on TIM-3 in the context of Alzheimer’s disease has been underway for approximately five years. Each phase of the experiments typically takes eight to nine months, reflecting the complexity and detail required to explore this potential treatment avenue.
What validation is needed before TIM-3 therapies can be used in humans?
Before TIM-3 therapies can be administered to humans, studies must first demonstrate efficacy and safety in appropriate animal models, particularly those that more closely mimic human Alzheimer’s disease. This validation process will help ensure that TIM-3 targeting can effectively halt the development of amyloid plaques and improve cognitive outcomes.
| Key Point | Details |
|---|---|
| Study Overview | Research shows that the TIM-3 molecule may provide a new strategy for treating Alzheimer’s Disease (AD) by enhancing microglial activity. |
| Role of TIM-3 | TIM-3 is a checkpoint molecule that inhibits microglia from clearing amyloid plaques, which are harmful in Alzheimer’s. |
| Alzheimer’s Disease Statistics | 90-95% of Alzheimer’s cases are late-onset, with TIM-3 linked to genetic susceptibility. |
| Microglia Function | Microglia serve as the brain’s immune cells and are crucial for both synapse pruning during development and clearing amyloid plaques. |
| Experiment Insights | Mice lacking TIM-3 showed improved plaque clearance and cognitive function. |
| Potential Therapy | The future treatment could involve anti-TIM-3 antibodies to enable microglia to clear plaques better. |
| Next Steps in Research | Aiming to test human anti-TIM-3 antibodies in Alzheimer’s mouse models. |
Summary
The TIM-3 Alzheimer treatment presents an innovative approach to combat one of the most challenging neurodegenerative disorders. By blocking the TIM-3 checkpoint molecule, researchers have found a way to enable microglia to effectively clear amyloid plaques from the brain. These findings suggest that repurposing existing anti-TIM-3 therapies may significantly enhance cognitive function in Alzheimer’s patients, offering hope for better treatment options in the future.