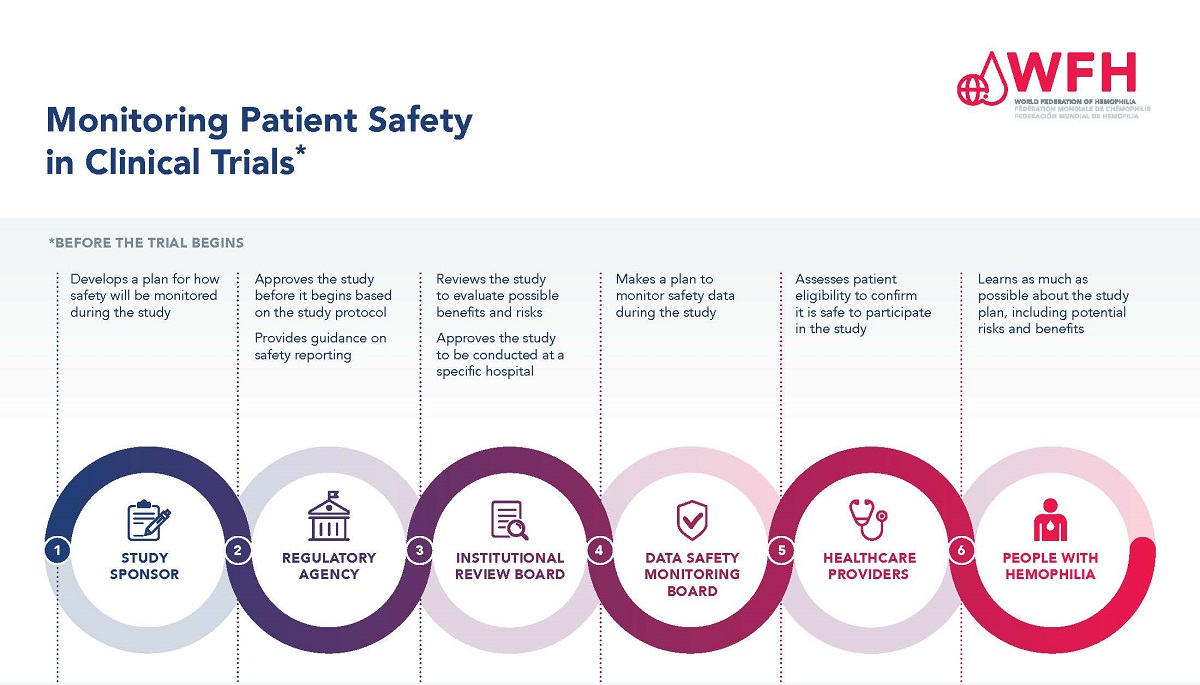
Patient safety in medical research is paramount to the integrity and ethical management of clinical trials. As funding for medical research dwindles, especially with the recent freeze on over $2 billion in federal research grants, the risks to research participants increase significantly. The involvement of Institutional Review Boards (IRBs) is crucial in maintaining oversight and ensuring the rights and protection of those who volunteer for studies. Enhanced mechanisms are needed, such as the SMART IRB system, which streamlines the collaborative process among multiple sites, however, this crucial oversight is jeopardized by funding challenges. Ensuring participant safety not only safeguards individual health but also upholds the credibility of the entire medical research framework, correlating closely with the principles set forth by NIH research grants and clinical trial oversight.
The safeguarding of individuals participating in clinical studies embodies the essence of ethical medical inquiry. In light of recent disruptions to research funding, the ability to protect the rights and safety of research subjects has come under threat. Oversight mechanisms, such as institutional review boards (IRBs), are vital for assessing research proposals and securing informed consent from participants while identifying potential risks. With increasing concerns about ethics and participant protections, it’s crucial to reflect on how funding cuts can hinder efforts to ensure that the research community adheres to strict safety protocols. A commitment to uphold participant welfare not only fosters trust but also enhances the validity and success of medical advancements.
The Importance of Patient Safety in Medical Research
Patient safety in medical research is paramount, a principle that ensures the health and welfare of research participants are prioritized at every step of the study process. With stringent requirements in place, such as the necessity for Institutional Review Board (IRB) approval, patient rights are protected through careful oversight and evaluation of research protocols. This mechanism allows for the identification and mitigation of potential risks, guaranteeing informed consent and that participants fully understand the implications of their involvement. By focusing on patient safety, researchers can not only uphold ethical standards but also maintain public trust in the medical research community.
Moreover, maintaining patient safety contributes to the integrity of the research outcomes. Studies that genuinely consider the welfare of participants are more likely to produce reliable and valid data, which can ultimately benefit society as a whole. When funding cuts disrupt these critical processes, as seen with recent federal research grant freezes, the potential for risks increases. Without adequate oversight, vulnerable populations could be exploited, and their well-being jeopardized, thus highlighting the urgent need for continued investment in patient safety measures within the realm of medical research.
The Effect of Funding Cuts on Medical Research Integrity
The disruption of funding for medical research has wide-reaching implications, particularly in terms of oversight mechanisms like the Institutional Review Boards (IRBs). With reduced resources, IRBs may face challenges in conducting thorough reviews and maintaining the standard levels of oversight required to protect research participants. This can lead to compromised patient safety, as fewer resources mean less capacity to monitor ongoing studies effectively. The potential for ethical breaches rises significantly, as the absence of robust oversight can pave the way for negligence in the treatment of research participants.
Furthermore, funding cuts can stall existing research projects or lead to a halt in new studies altogether. With major funding sources such as NIH research grants being curtailed, the ability to recruit diverse populations for clinical trials diminishes, which is crucial for ensuring that findings are applicable across different demographics. This reduction in research activity not only puts patient safety at risk but also hampers innovation in medical treatments that could save lives. As the landscape of medical research shifts, finding alternative funding solutions becomes imperative to safeguard the future of patient safety and the ethical conduct of research.
Empowering Researchers Through Ethical Oversight and Compliance Standards for Participants’ Rights and Safety
Frequently Asked Questions
How does medical research funding impact patient safety in medical research?
Medical research funding, particularly from agencies like the NIH, is crucial for ensuring patient safety in medical research. It provides the necessary resources for Institutional Review Board (IRB) oversight, which is vital for assessing risks and protecting research participants’ rights and welfare. Without adequate funding, the oversight mechanisms that safeguard patient participants in clinical trials may weaken, potentially leading to ethical breaches and increased risks.
What is the role of IRB patient protection in clinical trials?
IRB patient protection is essential in clinical trials as it ensures that research proposals are thoroughly reviewed for ethical compliance. The IRB evaluates the study design, informed consent processes, and participant risk assessments. This oversight helps mitigate potential harms and ensures that the rights of participants are respected, fostering trust and safety within the medical research environment.
How does clinical trial oversight promote patient safety in medical research?
Clinical trial oversight promotes patient safety by enforcing rigorous ethical standards and guidelines throughout the research process. Oversight bodies, including IRBs and monitoring committees, regularly review ongoing studies to ensure compliance with safety protocols. Their involvement helps identify and address any emerging risks to participants, ultimately prioritizing their welfare in the pursuit of scientific advancement.
What are research participant rights in the context of patient safety in medical research?
Research participant rights include informed consent, the right to withdraw from a study at any time, and access to information about the research and its potential risks and benefits. Protecting these rights is fundamental to patient safety in medical research, as it empowers participants to make informed choices and promotes ethical practices among researchers.
How do NIH research grants enhance patient safety in medical research?
NIH research grants enhance patient safety in medical research by funding studies that require robust ethical oversight. Grants often come with stipulations for IRB review and compliance with federal regulations, ensuring that research designs prioritize participant welfare. This funding supports the development of safe and effective treatments, aligning scientific goals with the protection of patient rights.
| Key Points | Description |
|---|---|
| Halting Funding | The Trump administration’s $2 billion freeze on federal research grants to Harvard disrupts research aimed at patient safety. |
| SMART IRB Role | A national system that enables oversight of medical research across multiple sites, critical for ensuring patient welfare. |
| Importance of IRBs | Institutional Review Boards (IRBs) review research to protect participants, ensuring compliance with ethical standards and regulations. |
| Impact of Cuts | Funding cuts negatively affect research participants and may lead to public skepticism about research integrity. |
| Historical Context | Past ethical violations underscore the need for rigorous oversight and the risks of research without adequate safeguards. |
Summary
Patient safety in medical research is critically undermined by funding cuts, as demonstrated by the Trump administration’s freeze on federal grants. This funding disruption has significant implications for patient rights and the ethical conduct of studies, highlighting the essential role of systems like SMART IRB in maintaining oversight and integrity across multiple research sites. Without robust financial support, the ability of Institutional Review Boards (IRBs) to ensure participant protection diminishes, leading to concerns about public trust in medical research. As history teaches us the devastating consequences of unchecked research practices, it remains paramount that we advocate for adequate funding to safeguard patient safety in medical research.