Gene therapy for hemophilia represents a groundbreaking advancement in treatment options for individuals affected by this condition. Recent innovations, such as Hemgenix, are providing new hope for patients like Terence Blue, who has struggled with hemophilia B throughout his life. This innovative hemophilia gene therapy aims to correct the deficiency of clotting factor IX in the body, offering a potentially permanent solution to manage bleeding episodes. By utilizing advanced genetic techniques, gene therapy benefits not only increase the production of this essential clotting factor but may also reduce the need for frequent injections. As more patients gain access to this transformative treatment for hemophilia B, the landscape of hemophilia management is set to change dramatically, ushering in a new era of possibility.
The world of hereditary bleeding disorders has seen remarkable shifts with the advent of gene-based treatments that aim to mitigate the effects of hemophilia. This genetic approach, often termed hemophilia gene therapy, leverages novel technologies to reintroduce functional genes that can effectively produce necessary clotting proteins in the bloodstream. For those battling the challenges of conditions such as hemophilia B, therapies like Hemgenix offer an invigorating prospect of reducing or even eliminating the reliance on traditional prophylactic treatments. The potential advantages of gene therapy include increased production of clotting factor IX, greatly improving the quality of life for patients. As we delve deeper into the implications of these advancements, the future appears increasingly bright for individuals and families grappling with the realities of hemophilia.
Understanding Gene Therapy for Hemophilia
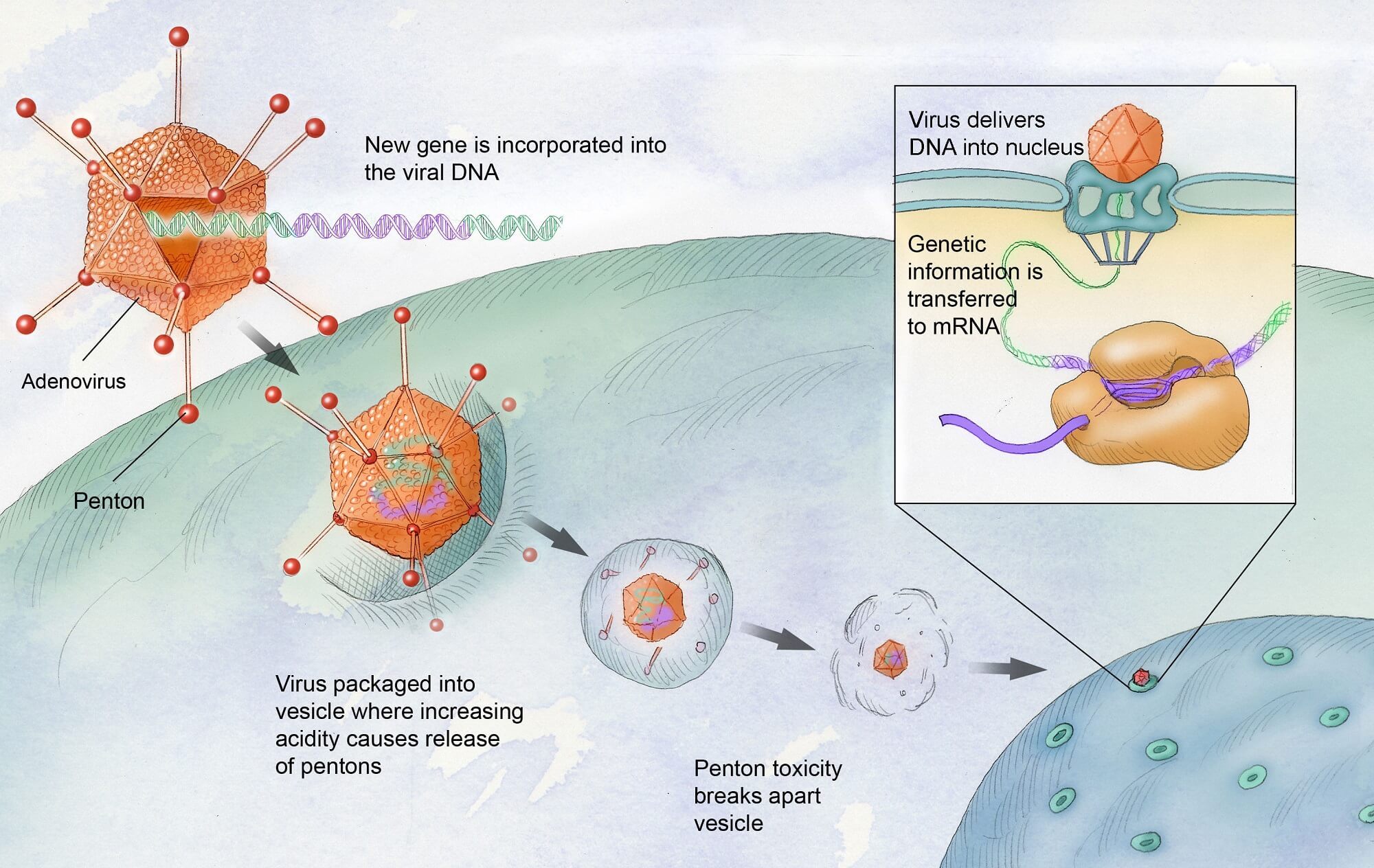
Gene therapy represents a groundbreaking approach in the treatment of hemophilia, particularly hemophilia B, a condition characterized by a deficiency in clotting factor IX. The therapy aims to address the root cause by delivering a corrected gene directly into the patient’s cells, effectively producing the missing clotting factor within the liver. Hemgenix, the first FDA-approved gene therapy for hemophilia B, exemplifies this innovative method, providing patients like Terence Blue the potential for a significant reduction in their reliance on regular infusions of factor IX. This approach has opened up a new frontier in hemophilia management, transitioning from symptomatic treatment to a potential permanent solution.
The benefits of gene therapy extend beyond merely reducing the frequency of injections; they include the promise of improved quality of life and decreased anxiety for patients. Traditional treatments for hemophilia often involve multiple painful injections, not to mention the constant worry of spontaneous bleeding. With gene therapy, patients may achieve long-term alleviation from these daily concerns. Reports have shown that a vast majority of trial participants who received Hemgenix experienced a sustained increase in their factor IX production, significantly changing their treatment landscape. As more patients become aware of these advancements, the discussion around gene therapy for hemophilia is becoming increasingly optimistic.
The Promise and Progress of Hemgenix
Hemgenix represents a landmark achievement in hemophilia research and treatment, illustrating the potential for gene therapy to transform patient outcomes. As the first approved therapy targeting hemophilia B, Hemgenix has been a part of the larger movement towards precision medicine, where treatments are tailored to address specific genetic mutations. The initial success observed in clinical trials has sparked excitement within the medical community, with factored filings indicating that 94% of participants continued to show elevated levels of clotting factor IX after three years. This statistic alone suggests that Hemgenix could fundamentally change the pharmacological landscape for hemophilia patients.
Beyond clinical efficacy, Hemgenix has also ignited conversations about future innovation in the field. Its application could pave the way for additional therapies targeting both hemophilia A and other related bleeding disorders, showcasing the versatility of genetic approaches in medicine. However, these advancements also bring about challenges, including access to treatment and financial implications. At a listed price of $3.5 million, the discussion surrounding the affordability of such groundbreaking therapies is critical, prompting healthcare systems to reassess how they can support widespread access without compromising patient care.
Market Dynamics Affecting Gene Therapies
The integration of gene therapies like Hemgenix into clinical practice is influenced not just by scientific breakthroughs but also by the economic landscape. As healthcare providers and patients celebrate the efficacy of these treatments, there are compelling market forces at play that could impact their availability. For instance, the high costs associated with developing and bringing gene therapies to market mean that pricing and insurance reimbursement become critical factors affecting patient access. With some drug manufacturers pulling back their products due to perceived cost inefficiencies, it becomes essential to engage in strategic discussions about how to ensure that effective therapies reach those in need.
Moreover, patient acceptance remains a key hurdle in the transition to gene-based therapies. Despite the promise held by approaches such as Hemgenix, there can be skepticism among patients regarding new treatments, particularly those involving genetic modification. This cautious approach is understandable given the historical context of emerging therapies. Education and awareness campaigns are paramount to dispel fears and provide a more profound understanding of the potential benefits of hemophilia gene therapy. Ultimately, navigating these market dynamics will define not only the future of hemophilia treatments but also the broader journey towards the acceptance and integration of gene therapies into standard healthcare practices.
Living with Hemophilia and the Role of Gene Therapy
For many individuals diagnosed with hemophilia, life can be fraught with unpredictability and challenges related to bleeding episodes. Daily treatments with clotting factors, especially for those with severe forms like hemophilia B, can dominate schedules and social interactions. For patients like Terence Blue, the introduction of gene therapy has sparked newfound hope. This innovative treatment could diminish the need for frequent infusions, promoting not only better physical health but also enhancing mental and emotional well-being. Gene therapy could potentially allow patients to engage in more activities without the constant fear of bleeding complications.
As discussions about gene therapy mature, there’s a growing recognition of the social implications of these treatments. The psychological burden that accompanies hemophilia—stemming from both the physical limitations and societal misunderstandings—can be alleviated through advancements like Hemgenix. Patients are encouraged to see life beyond the confines of their condition, leading to enriched social interactions and improved quality of life. The emergence of gene therapy represents not just a medical advancement but a holistic shift in the perception and management of hemophilia, offering a brighter future for patients.
Future of Gene Therapy and Clotting Factor Production
The introduction of Hemgenix has initiated a transformative era in the management of hemophilia B, particularly with respect to the production of clotting factor IX. As the therapy targets the liver for the endogenous production of this factor, patients can potentially experience life with significantly fewer bleeding risks. Future developments may continue to optimize gene therapies, improving their efficacy and safety profiles while allowing for broader applications beyond hemophilia. The hope for patients is that further research might lead to therapies that offer longer-lasting effects and even cures for various types of bleeding disorders.
Moreover, the ongoing pursuit of innovative gene therapy techniques could provide insight into similar treatments for related conditions. Lessons learned from hemophilia treatment using gene therapy could be applied to develop solutions for other inherited diseases characterized by gene mutations. Collaboration among researchers, healthcare professionals, and patients is essential in this pursuit, as it ensures that the needs of affected individuals are prioritized. Ultimately, the expanding possibilities associated with clotting factor production through gene therapy are paving the way for a future where living with hemophilia is defined by opportunity rather than limitation.
Challenges and Ethical Considerations in Gene Therapy
While the advancements in gene therapy for hemophilia, like Hemgenix, are promising, they also introduce a complex array of challenges and ethical considerations. The high cost of gene therapies raises critical questions about affordability and equitable access. If treatments remain prohibitively expensive, there exists a risk that only a subset of patients will benefit, perpetuating inequalities within the healthcare system. Ethical discussions must address how society can balance innovation with fairness in medical access, ensuring that all patients who could benefit from these groundbreaking treatments can attain them.
Additionally, ethical concerns surrounding the long-term effects of gene therapy must be explored. As gene alteration techniques progress, questions regarding genetic modification and its implications for future generations surface. Stakeholders must engage in transparent discussions about the boundaries and responsibilities that come with our growing understanding of genetic science. While the prospects of hemophilia gene therapy herald unprecedented hope, we must remain vigilant about the ethical landscape it creates, ensuring that all advancements are made responsibly and with the well-being of patients at the forefront.
Advancements in Hemophilia Care Through Research
The rapid pace of research in gene therapy is ushering in a new era for hemophilia care, marked by an increasing number of innovations. The success of therapies like Hemgenix not only validates the scientific approach but also encourages subsequent investigations into other forms of gene therapy. With clinical trials expanding, researchers are discovering new methodologies and combinations of treatments that could yield even more effective solutions for patients. This momentum in research is critical as it lays the foundation for future breakthroughs that could transform hemophilia treatment comprehensively.
Furthermore, collaboration among academic institutions, pharmaceutical companies, and advocacy groups is pivotal in catalyzing research advancements. These partnerships help expedite the transition from laboratory successes to real-world applications. Patients who once relied solely on traditional treatments are now seeing pioneers in gene therapy emerging from collaborative efforts, enhancing the potential for novel therapies tailored to specific genetic needs. As the field evolves, we can anticipate a surge of opportunities for patients to benefit from cutting-edge research focused on improving the quality of life for those living with hemophilia.
Patient Experiences and the Impact of Gene Therapy
The personal narratives of patients who have received gene therapy for hemophilia, such as Terence Blue, amplify the significance of this treatment in daily life. Their first-hand experiences illustrate not only the medical benefits but also the emotional relief that comes from undergoing a transformative therapy. The ability to heal from injuries quickly and enjoy life without the burden of routine factor IX infusions represents a significant milestone for patients who have lived under constant fear of bleeding episodes. These powerful stories highlight the importance of patient-centered care and the profound impacts that effective treatments can have on individual lives.
Moreover, these testimonials catalyze broader conversations about gene therapy’s role in society. The stories of individuals reclaiming their lives and embarking on newfound freedom serve as driving forces in advocacy efforts, encouraging others within the hemophilia community to engage with healthcare providers about the advancements in treatment options. By sharing their journeys, patients also foster a deeper understanding of the complexities involved in living with hemophilia, thereby humanizing the statistics surrounding treatment efficacy and access. Ultimately, the continuation of such narratives is paramount to promoting awareness and fostering a supportive environment for those affected by hemophilia.
Frequently Asked Questions
What is hemophilia gene therapy and how does it work?
Hemophilia gene therapy involves delivering a corrected copy of the gene responsible for producing clotting factor IX, which is deficient in hemophilia B patients. The recently approved gene therapy, Hemgenix, utilizes a viral vector to transport the corrected gene to liver cells, enabling them to produce sufficient amounts of this essential clotting factor, thereby reducing the risk of bleeding.
What are the benefits of gene therapy for hemophilia, specifically Hemgenix?
Gene therapy for hemophilia, like Hemgenix, offers several significant benefits, including the potential to alleviate the need for regular injections of clotting factor IX. Patients may experience improved quality of life with fewer spontaneous bleeds, reduced hospital visits, and the possibility of long-lasting effects that may minimize or eliminate their bleeding episodes.
How effective is Hemgenix as a treatment for hemophilia B?
Hemgenix has shown promising results, with nearly 94% of patients in clinical trials not requiring factor IX prophylaxis three years after treatment. This indicates that the therapy effectively enables the body to produce sufficient clotting factor IX, thus reducing the frequency of bleeding episodes.
What is the cost of gene therapy for hemophilia B and how is it covered by insurance?
The list price for Hemgenix is approximately $3.5 million, although most insurance companies negotiate lower rates. While the high upfront cost is a barrier for many, it may be offset by long-term savings on the costs associated with regular factor IX treatments.
What challenges does gene therapy for hemophilia face in the market?
Despite the potential of gene therapy for hemophilia, challenges include high treatment costs, market acceptance, and the need for patient education. Additionally, limited patient populations and pricing strategies can impact the availability and viability of these therapies.
Who is an ideal candidate for hemophilia gene therapy?
Candidates for hemophilia gene therapy, such as Hemgenix, typically include individuals diagnosed with hemophilia B who experience severe bleeding episodes or require frequent injections of clotting factor IX. A thorough evaluation by a healthcare provider is necessary to determine if gene therapy is the right option based on individual health and medical history.
Are there any side effects associated with gene therapy for hemophilia?
Common side effects of gene therapy for hemophilia may include mild liver enzyme elevations and other transient effects related to the infusion process. Most patients, however, report minimal complications, and careful monitoring by healthcare professionals helps manage any adverse effects that may arise.
What is the long-term outlook for patients undergoing gene therapy for hemophilia?
Patients who undergo gene therapy for hemophilia, such as with Hemgenix, may see significant long-term benefits, including sustained production of clotting factor IX and reduced dependence on regular factor infusions. While labeled as not a definitive cure, results have shown that many patients can live with minimal or no bleeds post-therapy.
How does gene therapy for hemophilia differ from traditional treatments?
Unlike traditional treatments which require regular infusions of clotting factor IX, gene therapy aims to correct the underlying genetic defect with a single infusion that can enable long-term production of the clotting factor. This revolutionary approach seeks to provide a more permanent solution compared to the ongoing management of hemophilia with regular treatments.
What is the process of receiving gene therapy for hemophilia like?
Receiving gene therapy for hemophilia typically involves an outpatient procedure where the patient is administered the therapy through an intravenous infusion. The process is closely monitored by healthcare professionals to ensure safety and effectiveness, and most patients can return home shortly after the procedure.
| Key Point | Details |
|---|---|
| Introduction of Gene Therapy | Terence Blue was the first patient in New England to receive Hemgenix, a gene therapy for hemophilia B, at Brigham and Women’s Hospital. |
| Living with Hemophilia | Patients with hemophilia often require frequent injections of clotting factor and must be careful to avoid injuries. |
| Functionality of the Therapy | Hemgenix targets the liver and introduces a corrected gene to help produce clotting factor IX, which is deficient in hemophilia B. |
| Cost of Treatment | Each treatment of Hemgenix costs approximately $3.5 million, although the market dynamics can affect pricing. |
| Regulatory Approval | Hemgenix received FDA approval in November 2022 and has shown promising results in clinical trials. |
| Patient Outcomes | Patients treated with Hemgenix have shown significant improvements, with many not needing factor IX prophylaxis after treatment. |
| Challenges in Gene Therapy | High costs and market pressures can limit the availability and acceptance of gene therapies, despite their potential. |
Summary
Gene therapy for hemophilia is a groundbreaking development in treatment that provides new hope for patients like Terence Blue. This innovative approach offers a potential solution to the lifelong management of hemophilia B, easing the burden associated with frequent injections and the anxiety of bleeding. As gene therapies like Hemgenix evolve, they hold the promise of transforming how hemophilia is treated, aligning with the growing potential for more effective and enduring solutions in the field of genetic medicine.